Consort Flow Diagram And Checklist
The flow diagram displays the progress of all participants through the trial.
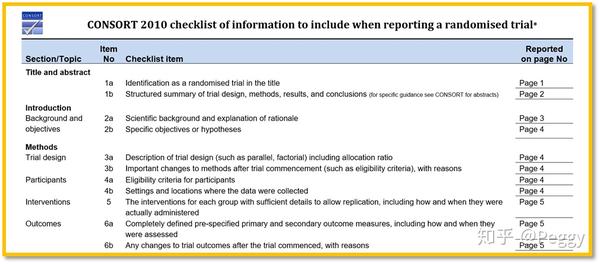
Consort flow diagram and checklist. The checklist items focus on reporting how the trial was designed analyzed and interpreted. The checklist items focus on reporting how the trial was designed analyzed and interpreted. Main consort 2010 articles extensions to consort if you are looking for a particular document and are not able to find it please contact us and we ll do our best to make it available. Awe strongly recommend reading this statement in conjunction with the consort 2010 explanation and elaboration for important clarifications on all t he items.
In addition to the checklist consort also encompasses a flow diagram which provides the reader with information about how the trial was conducted reporting enrolment allocation follow up and analysis of patients involved in the rct fig 1 5importantly the clinical. We strongly recommend that it is used in conjunction with the consort statement. Consort 2010 checklist of information to include when reporting a randomized triala. You can download the consort 2010 checklist here.
If relevant we also. This is a simple flow diagram showing how your study population was recruited and handled during the course of your study. Participant flow a diagram is strongly recommended. You must complete a flow diagram in order to be compliant with the consort 2010 standard.
The flow diagram displays the progress of all participants through the trial. Consort 2010 flow diagram. The consort extension for randomised pilot and feasibility trials is meant to provide reporting guidance for any randomised study in which a future definitive rct or part of it is conducted on a smaller scale regardless of its design eg cluster factorial crossover or the terms used by authors to describe the study eg pilot feasibility trial study. The consort explanation and elaboration document explains and illustrates the principles underlying the consort statement.
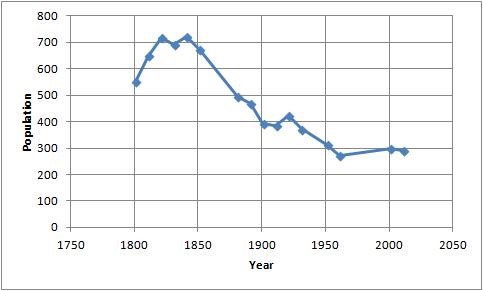
Analysed n excluded from analysis give reasons n lost to follow up give reasons n. Analysed n excluded from analysis give reasons n analysis. Flow diagram of the progress through the phases of a parallel randomised trial of two groups that is enrolment intervention allocation follow up and data analysis templates of the consort flow diagram are available in pdf and in ms word. The consort 2010 flow diagram.